Ferromagnetism is a property of a material in which particles called magnetic moments organize parallel to one another when there is a magnetic field. These particles remain in place even when the magnet is taken away. Magnetism occurs on an atomic level, with the field having a direct affect on the electrons in an atom. Electrons that spin in. Ferromagnetism A property exhibited by certain metals, alloys, and compounds of the transition (iron group), rare-earth, and actinide elements in which, below a certain temperature called the Curie temperature, the atomic magnetic moments tend to line up in a common direction.
- Ferromagnetism will provide a useful resource to any electrical engineer, physicist, researcher or designer, interested in the field of magnetics. Also of Interest Electromagnetics History, Theory, and Applications by Robert S. Elliott, UCLA A Classic Reissue in the IEEE Press Series on Electromagnetic Waves A handy reference for engineers.
- Ferromagnetism is a kind of magnetism that is associated with iron, cobalt, nickel, and some alloys or compounds containing one or more of these elements.
Paramagnetism
Ferromagnetism Examples
Paramagnetism is a form of magnetism whereby some materialsare weakly attracted by an externally applied magnetic field and form internalinduced magnetic fields in the direction of the applied magnetic field.
Materials or substances that display Paramagnetism arereferred to as paramagnetic. Some compounds and most chemical elements areparamagnetic under certain circumstances. However, true paramagnets displaymagnetic susceptibility according to the Curie or Curie-Weiss laws and exhibitParamagnetism over a wide temperature range.
Examples of paramagnetic substances/materials include:
- Aluminium
- Platinum
- Manganese
- Chromium
- Titanium
- Sodium
- Calcium
- Lithium
- Tungsten
- Niobium
- Copper chloride
- Crown glass
- Oxygen etc
Ferromagnetism Domain
What You Need ToKnow About Paramagnetic Material/substances
- They can be solid, liquid or gas.
- Every atom is a magnetic dipole having aresultant magnetic moment.
- Paramagnetic substances are weakly attracted byan external magnetic field.
- Paramagnetic substances lose their magnetism onremoval of the external magnetic field.
- They tend to move from the weaker to thestronger part of the field when placed in a non-uniform magnetic field.
- They get weakly magnetized in the same directionto that of the field in an external magnetic field.
- When a rod of a paramagnetic substance issuspended in a uniform magnetic field, it comes to rest with its lengthparallel to the direction of the field.
- Magnetic susceptibility for paramagneticsubstances is positive and small.
- The susceptibility of paramagnetic material decreaseswith an increase in temperature.
- The net magnetic moment of the paramagneticsubstance is zero because in absence of an external magnetic field, themagnetic moments of atomic magnets are randomly arranged.
- If a watch glass containing a small quantity ofparamagnetic liquid is placed on two unrelated magnetic poles, the liquid showsan elevation in the middle.
- If a magnetic field is applied to theparamagnetic liquid in one arm of U-tube, the liquid level in that arm rises.
- If paramagnetic gas is introduced between polepieces of magnet, it spreads in the direction of the magnetic field.
Diamagnetism
Diamagnetism is a very weak form of magnetism that isinduced by a charge in the orbital motion of electrons due to an appliedmagnetic field. This magnetism is nonpermanent and persists only in thepresence of an external field. The magnitude of the induced magnetic moment isvery small and its direction is opposite to that of the applied field.
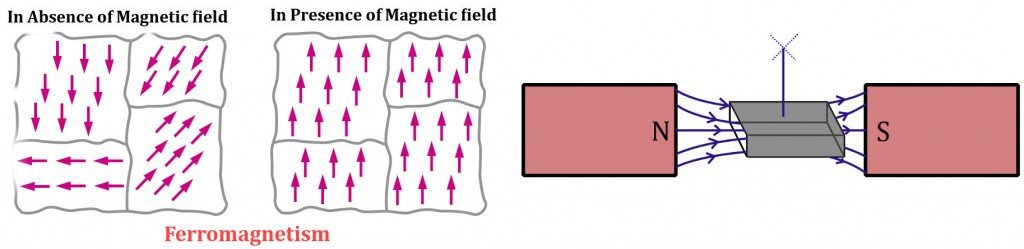
Diamagnetism is found in all materials but because it is soweak, it can be observed only when other types of magnetism are totally absenthence it is of no practical importance.
Examples of diamagnetic materials/substances include:
- Bismuth
- Antimony
- Copper
- Marble
- Zinc
- Graphite
- Silver
- Nitrogen
- Gold
- Quartz
- Mercury
- Water
- Alcohol
- Air
- Hydrogen etc
What You Need ToKnow About Diamagnetic Material
- They can be solid, liquid or gas.
- The magnetic moment of every atom of diamagneticsubstance/material is zero.
- Diamagnetic materials/substances are weaklyrepelled by an external magnetic field.
- Diamagnetic substances/material lose theirmagnetism on removal of the external magnetic field.
- Diamagnetic material/substance tends to movefrom the stronger to the weaker part of the field.
- Diamagnetic materials get weakly magnetized indirection opposite to that of the field.
- When a rod of diamagnetic substance/material issuspended in a uniform magnetic field, it comes to rest with its lengthperpendicular to the directions of the field.
- Magnetic susceptibility of diamagneticsubstance/material is negative.
- Temperature has no effect on diamagneticmaterial/substance.
- The net magnetic moment of diamagnetic substanceis zero in the absence of an external magnetic field.
- If a watch glass containing a small quantity ofdiamagnetic liquid is placed on two dissimilar magnetic poles, the liquid showsa depression in the middle.
- If a magnetic field is applied to a diamagneticliquid in one arm of U-tube, the liquid level in that arm is lowered.
- If diamagnetic gas is introduced between polepieces of magnet, it spreads at a right angle to the magnetic field.
Ferromagnetism
Ferromagnetism can be described as a physical phenomenon inwhich certain materials attain permanent magnetism or they acquire attractivepowers. It is also described as a process where some of the electricallyuncharged materials attract each other strongly. Ferromagnetism is a property that considersnot only the chemical composition of a material but also takes intoconsideration the microstructure and crystalline structure.
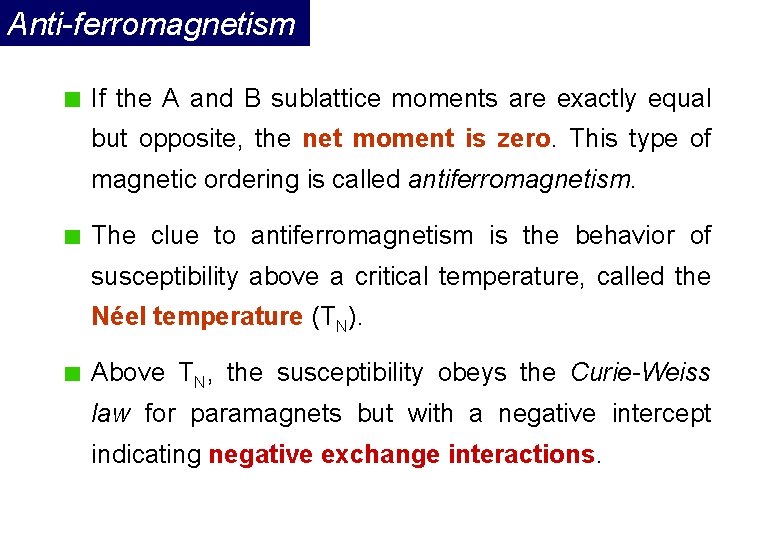

In a ferromagnetic material in the umagnetized state, atomicdipoles in small regions referred to as domains are aligned in the samedirection. Remote ndis based device driver. The domains exhibit a net magnetic moment even in the absence of anexternal magnetizing field.
However, the magnetic moments of neighboring domains areoriented in opposite directions. They cancel out and therefore the net magneticmoment of the material is zero. On application of external magnetic field,these domains all align themselves in the direction of the applied field. In this regard, the material is stronglymagnetized in a direction parallel to the magnetizing field.
Ferromagnetic materials can retain their magnetization whenthe external field is removed, as long as the temperature is below a criticalvalue, the Curie temperature. They arecharacterized by a large positive magnetic susceptibility.
Examples of Ferromagnetic substances/materials include:
- Iron
- Cobalt
- Nickel
- Gadolinium
- Metallic alloys
- Rare earth magnets
What You Need ToKnow About Ferromagnetic Substances/Material
- They are solid.
- Ferromagnetic substances/material consists of alarge number of small domains. The atomic magnets in one domain are aligned inthe same direction due to strong interaction.
- Ferromagnetic material/substance are stronglyattracted by external magnetic field.
- Ferromagnetic material/substances do not losemagnetism when the external magnetic field is removed i.e they are permanentmagnets.
- Ferromagnetic material tends to move from theweaker to the stronger part of the field.
- Ferromagnetic material/substance get stronglymagnetized in the same direction to that of the field.
- When a rod of a ferromagnetic substance issuspended in a uniform magnetic field, it comes to rest with its lengthparallel to the directions of the field.
- Magnetic susceptibility of ferromagneticmaterial/substance is positive and large.
- When heated above Curie temperature,ferromagnetic material/substance becomes paramagnetic.
- The net magnetic moment of a ferromagneticsubstance is zero because in the absence of an external magnetic field, themagnetic moments of domains are randomly arranged.
Also Read:Difference Between Permanent Magnet And Electromagnet
Difference Between Diamagnetic, Paramagnetic And Ferromagnetic Materials In Tabular Form

| BASIS OF COMPARISON | PARAMAGNETIC MATERIAL/SUBSTANCES | DIAMAGNETICMATERIAL/SUBSTANCES | FERROMAGNETIC MATERIAL/SUBSTANCES |
| Nature | They can be solid, liquid or gas. | They can be solid, liquid or gas. | They are solid. |
| Atoms | Every atom is a magnetic dipole having a resultant magnetic moment. | The magnetic moment of every atom of diamagnetic substance/material is zero. | They consists of a large number of small domains. The atomic magnets in one domain are aligned in the same direction due to strong interaction. |
| Attraction | They are weakly attracted by an external magnetic field. | They are weakly repelled by an external magnetic field. | They are strongly attracted by external magnetic field. |
| Lose Of Magnetism | They lose their magnetism on removal of the external magnetic field. | They lose their magnetism on removal of the external magnetic field. | They do not lose magnetism when the external magnetic field is removed i.e they are permanent magnets. |
| Movement In Magnetic Field | They tend to move from the weaker to the stronger part of the field when placed in a non-uniform magnetic field. | They tend to move from the stronger to the weaker part of the field. | They tend to move from the weaker to the stronger part of the field. |
| Magnetization | They get weakly magnetized in the same direction to that of the field in an external magnetic field. | They get weakly magnetized in direction opposite to that of the field. | They get strongly magnetized in the same direction to that of the field. |
| Suspension In Uniform Magnetic Field | When a rod of a paramagnetic substance is suspended in a uniform magnetic field, it comes to rest with its length parallel to the direction of the field. | When a rod of diamagnetic substance/material is suspended in a uniform magnetic field, it comes to rest with its length perpendicular to the directions of the field. | When a rod of a ferromagnetic substance is suspended in a uniform magnetic field, it comes to rest with its length parallel to the directions of the field. |
| Magnetic Susceptibility | Magnetic susceptibility for paramagnetic substances is positive and small. | Magnetic susceptibility of diamagnetic substance/material is negative. | Magnetic susceptibility of ferromagnetic material/substance is positive and large. |
| Temperature | The susceptibility of paramagnetic material decreases with an increase in temperature. | Temperature has no effect on diamagnetic material/substance. | When heated above Curie temperature, ferromagnetic material/substance becomes paramagnetic. |
| Net Magnetic Moment | The net magnetic moment of the paramagnetic substance is zero because in absence of an external magnetic field, the magnetic moments of atomic magnets are randomly arranged. | The net magnetic moment of diamagnetic substance is zero in the absence of an external magnetic field. | The net magnetic moment of a ferromagnetic substance is zero because in the absence of an external magnetic field, the magnetic moments of domains are randomly arranged. |
Related posts:
